Experimental Vivisection — N6 — Version2
An update on version 2 of the N6 plasmid experiment. This is a living document that will be updated as we get new data. This experiment is intended as a proof of concept for a possible future therapy. This experiment is in no way intended to cure or treat HIV: a cocktail of similar therapies, each delivering different antibodies, would be needed for a durable treatment. The production of antibodies using plasmids, if feasible, could greatly impact patient outcomes. Context This ‘Vector A’ was designed to express the gene for N6, an antibody that binds to the GP120 protein on HIV’s envelope. The binding action of N6 neutralizes the virus’s ability to infect cells, acting as both a prophylactic and treatment for those already infected. The dependent variable is the blood’s tendency to bind to GP120. The hypothesis is that there will be some binding in the samples taken prior to this injection with more binding following the hopeful transfection of the N6 gene. This is believed to be the first ever reported antibody gene delivery experiment in a human, but also the first reported human minicircle experiment, and the first time a self-editing I-SecI endonuclease cassette had ever been tested in a human. Safety A test injection was done nearly a week before the main injection. These two injections were roughly 1:14 in proportion. A small red bump materialized that was similar to the 2017 injection. No other symptoms seemed to occur. In retrospect, this perhaps should’ve given me pause, but linear thinking gave me some comfort: surely I could handle x14 of a small red bump. This was faulty logic: the immune system doesn’t necessarily respond in a linear fashion. The “test dose” could even prime the system for a more severe reaction later. Fortunately, the response to the primary injection was roughly linear. Inflammation broke out in my lower abdomen, and nearly a pea sized bit of flesh seemed to liquify under the clear bandage I kept over it. The plasmids we received were guaranteed to be close to the safety guidelines for human consumption, in terms of endotoxin present (<50 endotoxin units per milligram; <40 is the US FDA guideline). However, labs shouldn’t be trusted as absolute sources of truth, and my associates had to perform the additional step of purifying the plasmids. Following miniprep process for replication, an endotoxin wash and endotoxin screening was performed. The final 260/280 absorbance ratio was 1.89 to 1.87 according to Nanodrop [1.89, 1.89, 1.87]. Full dose: 10 micrograms of plasmid + 40 micrograms PEI + 11 microliters saline. Complexed for 30 minutes. Analysis In order to accurately quantify any results from this procedure, blood serum samples were taken before and after the attempt at transfection. Team planned on using N6 antibodies, obtained from the NIH, to establish a positive control using diluted suspensions. These suspensions would allow us to quantify the effect, rather than just determine if there was a relative effect between the before and after conditions. The independent lab contracted for this procedure was unable to establish a calibration curve or positive control with the materials supplied. [Western Blot data] Another trial is currently being run with another antibody standard to attempt to establish a positive control. While some of the antibody provided still remains after the failed procedures, my team has decided to pursue the production of the antibody using another third party lab. This process should facilitate future research -- we did get the last batch of N6 the NIH had to offer to non-profits -- but this decision has slowed the process considerably. Meanwhile, Team seeks experts in the domain of bnAbs for navigating the institutional circle-jerking protocols. For instance, we know some researchers have access to the gene sequences for ‘antibody anti-idiotypes’ that would speed up the Team’s production of N6 considerably, but the ones we’ve reached out to don’t seem particularly communicative. Hypotheses The present experiment sought to accomplish something novel: transfection of a broadly neutralizing antibody. The therapeutic possibly of plasmids is well enough established that the FDA and EU have guidelines for their production. The quantities of endotoxin present after the screen was performed were well below those thresholds. Claims:
- That the plasmid was not tainted with endotoxin, according to the endotoxin wash kit and subsequent analysis performed by Lonza.
- The plasmid and PEI, when injected independently, did not cause similar inflammation.
- Previous animal studies have used I-secI meganuclease in their plasmid constructs without notable inflammation
- The transfection produced malformed antibodies inside the cells
- The interaction between N6 antibodies and the virus
- The presence of I-secI meganuclease inside the cells
- A combination of factors the above along with the age of the PEI suspension
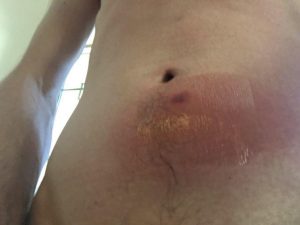 48 hours post injection of Vector A. Injection site is right below the belly button. There’s a clear bandage over the area, giving that weird sheen.I started developing a slight fever around this time. While I’m guessing the injection was partially to blame, I think I already had a throat infection developing from sharing perhaps a few too many spliffs in the days leading up to the injection.The inflammation shown above was further aggregated, I believe, by a long road trip in which the area was chronically squished from the sitting position.I started to feel shitty around this time. Besides a continuous flow of snot, bowel movements increased from around once a day to around thrice.
48 hours post injection of Vector A. Injection site is right below the belly button. There’s a clear bandage over the area, giving that weird sheen.I started developing a slight fever around this time. While I’m guessing the injection was partially to blame, I think I already had a throat infection developing from sharing perhaps a few too many spliffs in the days leading up to the injection.The inflammation shown above was further aggregated, I believe, by a long road trip in which the area was chronically squished from the sitting position.I started to feel shitty around this time. Besides a continuous flow of snot, bowel movements increased from around once a day to around thrice.
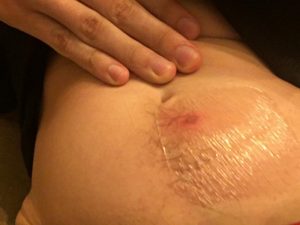 Six days after injection.
Six days after injection.

