
Unnatural Selection is a top-rated Netflix original miniseries documenting the beginning of our work, 2 years ago.



Unnatural Selection is a top-rated Netflix original miniseries documenting the beginning of our work, 2 years ago.

A group of Canadian filmmakers doing a feature-length documentary called Citizen Bio came to our lab to film our work for a few days.






There is more optimism than ever that researchers are on the path to developing vaccines and antibodies that can help stem HIV’s persistent spread.
“A new era. An exciting time. More optimism than ever before.” These are just some of the ways researchers are describing the current state of play in HIV vaccine and antibody research.
There is very exciting new research that gives us great hope that we are making substantial progress with vaccines and antibodies. We have better vaccine antigens in the last few years than we’ve ever had, and also better vaccine platforms. An effective vaccine is likely, and we’re on that path.
John Mascola, director of the Vaccine Research Center (VRC) at the U.S. National Institute of Allergy and Infectious Diseases.
We will achieve new breakthroughs in science and medicine and I see what they’re doing. I see it. They show me the things we’re doing in our country today. There’s never been anything like it. We will be ending the AIDS epidemic shortly in America.
President Trump at a speech Aug 1 2019 in Cincinnati, Ohio [source]
In 2016 NIH scientists discovered the N6 antibody in blood samples collected from an HIV-resistant patient.
Scientists from the National Institutes of Health have identified an antibody from an HIV-infected person that potently neutralized 98 percent of HIV isolates tested, including 16 of 20 strains resistant to other antibodies of the same class. The remarkable breadth and potency of this antibody, named N6, make it an attractive candidate for further development to potentially treat or prevent HIV infection, say the researchers.
“NIH Scientists Identify Potent Antibody that Neutralizes Nearly All HIV Strains.” NIH Newsroom. [source]
Humans naturally develop antibodies as a response to disease. These antibodies selectively bind to and disarm pathogens like HIV. Your natural immunity is made possible by antibodies.
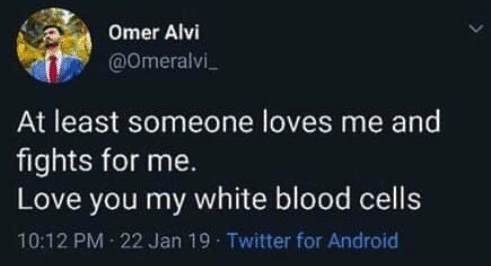
Broadly neutralizing antibodies (bNAbs) are rare. It takes three to three to five years of chronic stimulation by a virus before the immune system can generate potent and broadly cross-reactive antibody. And even then, most people still cannot make them.
Minicircle.HIV is a project to develop a safe, reliable means to share the genetic information encoding immunity from person to person.
HIV is coated with bulky sugar molecules that themselves are not immunogenic and largely deflect immune responses mounted against the virus.
“Antibodies prefer to see proteins. It’s very hard for an antibody to navigate that glycan shield, find a protein, and neutralize.”
John Mascola, director of the Vaccine Research Center (VRC) at the U.S. National Institute of Allergy and Infectious Diseases.
Despite its glycan shield, HIV’s outer protein known as Envelope (Env) actually has many sites of vulnerability.
“Virtually the entire Env can be targeted [by antibodies].”
Andrew Ward, a professor at Scripps Research in La Jolla, CA, and a principal investigator of IAVI’s Neutralizing Antibody Center (NAC).
Another reason HIV is hard to cure is because of its latent reservoir. HIV infects CD4 white blood cells by penetrating the cell surface and injecting its genome. This genome contains instructions for editing the host cell’s genome to include HIV production.
Even if all HIV particles can be removed from the blood using traditional anti-retroviral therapy or broadly neutralizing antibodies, the CD4 cells themselves are still infected, and would either need to be removed, or gene edited.
The process of removing the latent reservoir has only been documented in humans once. Administration of the antibody Nivolumab to an HIV-infected patient in France in 2017 showed for the first time a dumping of the latent reservoir.
This is the first demonstration of this mechanism working in humans. It could have implications for HIV patients, both with and without cancer, as it can work on HIV reservoirs and tumor cells independently. The absence of side effects in this patient is also good news, and suggests this could be an optimum treatment for HIV-infected patients with cancer.
Jean-Philippe Spano, MD, PhD, head of medical oncology at Pitie-Salpetriere Hospital AP-HP in Paris
A fourth reason curing HIV is so difficult is because of gp120. Even if all HIV particles are removed, and the latent reservoir dumped, gp120 will still be present in all bodily tissues. gp120 is a neurotoxin and prevents stem cells in the brain from producing new brain cells. gp120 is difficult for the body to get rid of and been proven to aide infection by inducing apoptosis (cell death), mitochondrial death, and oxidative stress. [source]
The production of unnecessarily large amounts of intracellular and extracellular protein aggregates is associated with other diseases, like Alzheimer’s and SENS Research Foundation has defined it as a hallmark of aging.
A fourth reason why curing HIV is so difficult is because of neuro-AIDS, also called HIV-associated neurocognitive disorder (HANDS). HIV infects the brain almost immediately upon arrival to the body, and causes brain cells to secrete neurotoxins, causing brain death. Because of the blood-brain barrier (BBB), most antibodies cannot pass into the brain. Meaning it is very unlikely that any vaccine ever produced could end neuro-AIDS for someone already infected. To make matters worse, anti-retroviral drugs like efavirenz are toxic to the central nervous system. This may be behind the cause of why the drug causes intense dreaming and is used for recreational purposes. [source]
After decades of disappointing results from clinical trials, there are now more funded clinical trials than ever searching for a cure. Many of the traditional vaccine approaches will not work, and trials using old antibodies like VRC01 can also not be expected to work.
However, new research with broadly neutralizing antibodies suggests a cure may be found.
“When it comes to getting a good clinical agent, we’re going to need a combination.”
Bette Korber, computational biologist at Los Alamos National Laboratory.
Because HIV is a diverse and rapidly mutating virus, combinations of antibodies working in tandem are necessary in order to create a vaccine that would truly neutralize all strains.
One interesting development in HIV bNAbs science is the development of multivalent antibodies – synthetic antibodies which combine the best attributes of multiple antibodies into one single antibody. Multivalent antibodies are significantly more potent.
Of the multivalent antibodies, 10E8v4-5R+100cF is one of the best. It neutralizes HIV particles from every clade and leaves almost none to spare.
However, the 10E8 backbone has been shown in human studies to produce a poorly understood inflammatory response, something very rare for antibodies.

David Ishee: “If you just do nothing, then it continues. From the point where we have the ability and choose not to exercise, all the suffering that comes after is the product of our choice. We chose to let them suffer.”
Moreover, a live-stream shocked the whole nation last year. A HIV positive person did a experiment of genetic modification on his own body, hoping that he could get cured. No medical expert was involved there.
Tristan Roberts: Our project is — because — it’s looking like it would be affordable for any one to be able to modify their genetic behavior.
Mr. Tristan Roberts works as writer in the field of new technology. He takes drug not to increase the number of HIV virus. Considering the cost of drug and side effects that last for the rest of his life, he wanted to be cured completely. He came across an article of NIH which was published 2 years ago. And it mentioned the existence of a gene that attacks HIV.
Tristan Roberts: “One on this side and one on this side. That was it.”
To be able to access the treatment using this gene, people have to wait years for clinical trials. So, Mr. Roberts decided to experiment using his own body. A group of individual researchers helped him to get the gene.
Tristan Roberts: “There is many promising treatments that maybe it worked in animals or maybe it worked in few humans. It never ends up reaching people who need the treatment. The possibility of doing good for thousands of people out weighted possibility of something bad happening to me.”
Since the experiment more than half a year ago, the effect of reducing HIV virus has not been confirmed. Also, so far, side effects have not appeared.

Here at Minicircle.HIV we believe that the true enemy is not each other: it is entropy, disease, and death. These are the ultimate underlying burdens that we as global humanity are capable of facing and acting.

“Compassion hurts. When you feel connected to everything, you also feel responsible for everything. You can not turn away. Your destiny is bound to the destinies of others. You must either learn to carry the universe or be crushed by it. You must grow strong enough to love the world, yet empty enough to sit down at the same table with its worst horrors. To seek enlightenment is to seek annihilation, rebirth, and the taking up of burdens. You must come prepared to touch and be touched by each and every thing in heaven and hell. I am one with the universe and it hurts.”
Andrew Boyd. (2001). Daily Afflictions: The Agony of Being Connected to Everything in the Universe.
An update on version 2 of the N6 plasmid experiment. This is a living document that will be updated as we get new data.
This experiment is intended as a proof of concept for a possible future therapy. This experiment is in no way intended to cure or treat HIV: a cocktail of similar therapies, each delivering different antibodies, would be needed for a durable treatment.
The production of antibodies using plasmids, if feasible, could greatly impact patient outcomes.
Context
This ‘Vector A’ was designed to express the gene for N6, an antibody that binds to the GP120 protein on HIV’s envelope. The binding action of N6 neutralizes the virus’s ability to infect cells, acting as both a prophylactic and treatment for those already infected.
The dependent variable is the blood’s tendency to bind to GP120. The hypothesis is that there will be some binding in the samples taken prior to this injection with more binding following the hopeful transfection of the N6 gene.
This is believed to be the first ever reported antibody gene delivery experiment in a human, but also the first reported human minicircle experiment, and the first time a self-editing I-SecI endonuclease cassette had ever been tested in a human.
Safety
A test injection was done nearly a week before the main injection. These two injections were roughly 1:14 in proportion.
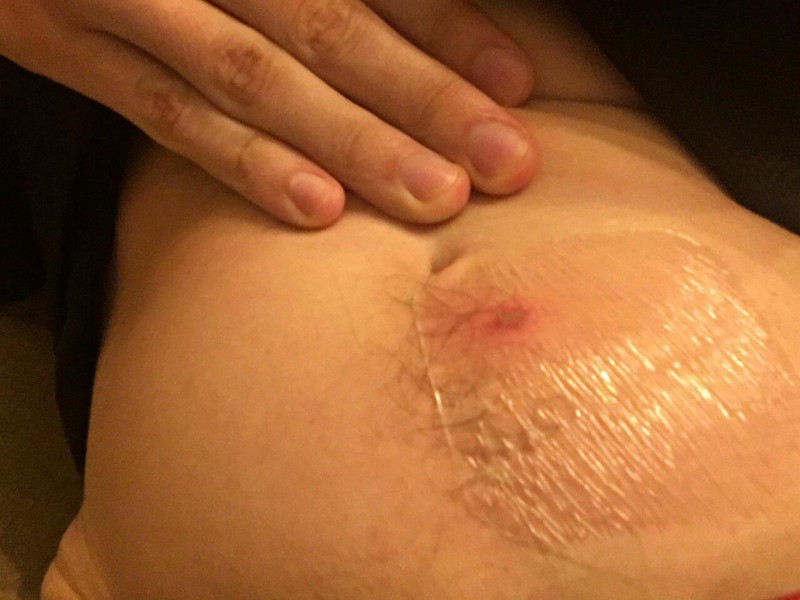
A small red bump materialized that was similar to the 2017 injection. No other symptoms seemed to occur. In retrospect, this perhaps should’ve given me pause, but linear thinking gave me some comfort: surely I could handle x14 of a small red bump.
This was faulty logic: the immune system doesn’t necessarily respond in a linear fashion. The “test dose” could even prime the system for a more severe reaction later. Fortunately, the response to the primary injection was roughly linear. Inflammation broke out in my lower abdomen, and nearly a pea sized bit of flesh seemed to liquify under the clear bandage I kept over it.
The plasmids we received were guaranteed to be close to the safety guidelines for human consumption, in terms of endotoxin present (<50 endotoxin units per milligram; <40 is the US FDA guideline). However, labs shouldn’t be trusted as absolute sources of truth, and my associates had to perform the additional step of purifying the plasmids. Following miniprep process for replication, an endotoxin wash and endotoxin screening was performed. The final 260/280 absorbance ratio was 1.89 to 1.87 according to Nanodrop [1.89, 1.89, 1.87].
Full dose: 10 micrograms of plasmid + 40 micrograms PEI + 11 microliters saline. Complexed for 30 minutes.
Analysis
In order to accurately quantify any results from this procedure, blood serum samples were taken before and after the attempt at transfection. Team planned on using N6 antibodies, obtained from the NIH, to establish a positive control using diluted suspensions. These suspensions would allow us to quantify the effect, rather than just determine if there was a relative effect between the before and after conditions.
The independent lab contracted for this procedure was unable to establish a calibration curve or positive control with the materials supplied. [Western Blot data] Another trial is currently being run with another antibody standard to attempt to establish a positive control. While some of the antibody provided still remains after the failed procedures, my team has decided to pursue the production of the antibody using another third party lab. This process should facilitate future research — we did get the last batch of N6 the NIH had to offer to non-profits — but this decision has slowed the process considerably.
Meanwhile, Team seeks experts in the domain of bnAbs for navigating the institutional circle-jerking protocols. For instance, we know some researchers have access to the gene sequences for ‘antibody anti-idiotypes’ that would speed up the Team’s production of N6 considerably, but the ones we’ve reached out to don’t seem particularly communicative.
Hypotheses
The present experiment sought to accomplish something novel: transfection of a broadly neutralizing antibody. The therapeutic possibly of plasmids is well enough established that the FDA and EU have guidelines for their production. The quantities of endotoxin present after the screen was performed were well below those thresholds.
Claims:
Thus, we are seeking additional comment on, or thoughtful refutation of, these possible hypotheses on the cause of the observed reaction:
Notes: In vitro expression of N6, along with binding to GP120
Vector A
Vector A is a plasmid which when transformed into a human cell, should self-edit into a minicircle via the I-SecI homing endonuclease transgene sourced from Saccharomyces cerevisiae. No homing sequence for this nuclease exist in the human or mouse genome, so editing of chromosomal DNA is almost impossible. The plasmid form is ephemeral but the minicircle form is a long lasting, high producing form of gene delivery. Efficacy was seen both in adult mouse studies currently pending publication, as well as in mouse, porcine, and fish embryos.
The purpose of investigating Vector A is because the cost of production and purification is exponentially lower than Vector B. I-SecI endonuclease may be immunogenic.
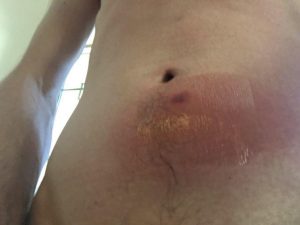
48 hours post injection of Vector A. Injection site is right below the belly button. There’s a clear bandage over the area, giving that weird sheen.I started developing a slight fever around this time. While I’m guessing the injection was partially to blame, I think I already had a throat infection developing from sharing perhaps a few too many spliffs in the days leading up to the injection.The inflammation shown above was further aggregated, I believe, by a long road trip in which the area was chronically squished from the sitting position.I started to feel shitty around this time. Besides a continuous flow of snot, bowel movements increased from around once a day to around thrice.
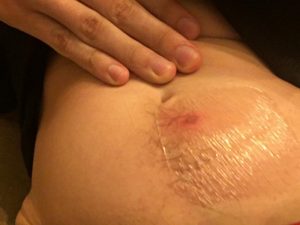
Six days after injection.